EXTRACELLULAR VESICLES
THE ORIGIN AND PHENOTYPING OF EXTRACELLULAR VESICLES
Nano-sized extracellular vesicles (EVs) released by various cell types play important roles in a plethora of (patho) physiological processes and are increasingly recognized as biomarkers for diseases. Moreover, engineered EV and EV inspired liposomes hold great potential as drug delivery vehicles. EVs are heterogeneous in composition and size, ranging from approximately 30-1000 nm, with the vast majority <200 nm in size. As determined by their biogenesis, the three main classes of EVs are exosomes, microvesicles, and apoptotic bodies. In contrast to microvesicles, which are generated by budding from the plasma membrane, exosomes are derived from the endolysosomal pathway, and fall in the size range of 30-150 nm.
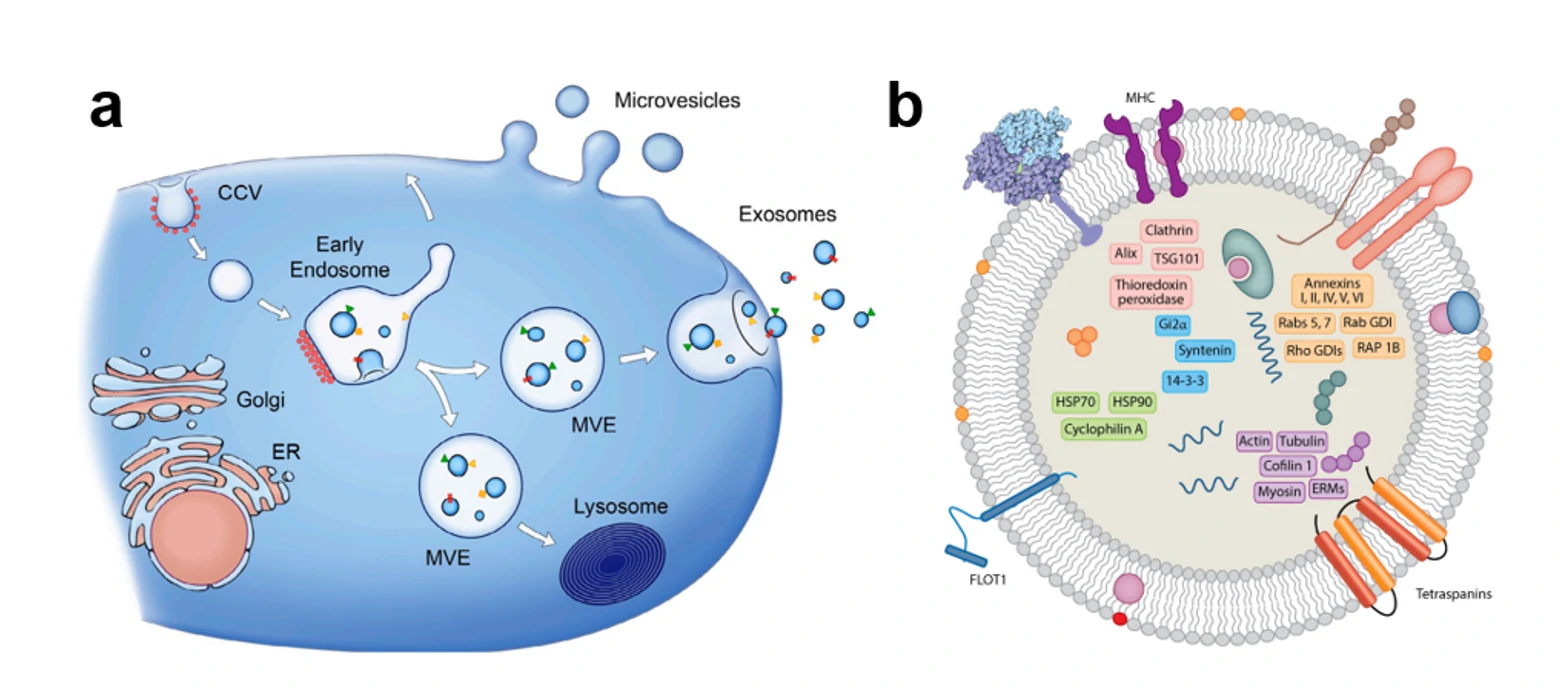
Figure 1. The secretory pathway and schematic structure of the extracellular vesicles.
EVs released from different cell types (and even from a single cell type) are shown to be highly heterogeneous in size and nanostructure, a specific vesicle subtype could be solely responsible for a particular function. The function of EVs are typically evaluated on RNA level (qPCR) and protein level (ELISA, IF, Western blots). However, these ensemble-averaged techniques could not discriminate the distinct subset from other abundant EVs. Therefore, a sensitive, specific, and rapid methodology is urgently needed to measure the abundance of these specific markers on EVs at the single-particle level. However, the tiny size, heterogeneity, low refractive index as well as lacking of distinct markers make it extremely challenging for the single-particle analysis of EVs. The gold standard for nanoparticle measurement has been electron microscopy (EM), which can determine both the size and morphology of the particles. However, the sample preparation steps and imaging techniques require dehydration, chemical fixation and/or staining of the biological specimens, which may alter the morphology of the samples. While for biological particles like EVs, cryo-electron microscopy (cryo-EM) is used to preserve the natural morphology of particles. But its routine application is prohibited due to the extremely high price and limited statistical power. Nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing (TRPS) are frequently used techniques to determine the size of EVs. The minimum detectable vesicle size are 70-90 nm and 70-100 nm, respectively. Unfortunately, none of these methods on its own have the ability to reveal the biochemical properties of EVs. Flow Cytometry (FCM) is a well-established technique for high-throughput, multi-parameter, and quantitative analysis of individual cells and microscopic particles in aqueous suspension. Although FCM has been applied to analyze surface proteins of individual EVs using fluorescence threshold triggering, the minimum detectable vesicle sizes are 150-190 nm for dedicated FCM and 270-600 nm for conventional FCM, which is far from satisfaction. Flow NanoAnalyzer opens a new avenue for single EVs detection, especially for the detection of exosomes —- the subset of EVs with size ranging from 30-150 nm. Most of the data are unpublished, please keep an eye on the website.