Measurement of complete lentivirus particles
Author: admin Date: February 22, 2024
Lentivirus is a gene vector developed from HIV-1 (human immunodeficiency type I virus), composed of a lipid membrane, capsid, and internal RNA genome with an average diameter of 80-120 nm. Lentiviral vectors now play a critical role in the field of cell and gene therapies, especially chimeric antigen T-cell (CAR-T) therapy. Currently, the most frequently used methods for titration of lentivirus vectors are:
1) Transducing Units (TU): Based on the capability of transducing a cell and expressing the transgene.
2) Copy Number Determination: Based on real-time quantitative PCR (qPCR).
3)Analysis of Viral p24 Concentration: Using enzyme-linked immunosorbent assay (ELISA).
Each method provides separate important data about a lentiviral sample. Nano-flow cytometry can be employed to describe all these lentiviral characteristics simultaneously at a single particle level. Additionally, the use of the Flow NanoAnalyzer on lentiviral products identifies both mature lentiviruses and contaminant unincorporated components.
Multiple components coexist in lentiviral samples, such as mature virions, partially assembled virions, free nucleic acids, etc. Analysis by nano-flow cytometry can give a thorough description of the active population of lentivirus presenting VSV-G for cellular recognition and infectivity, and internal nucleic acid cargo for activity. This data is highly quantitative, achieved rapidly, and simultaneously measures the other immature particles to determine product purity.
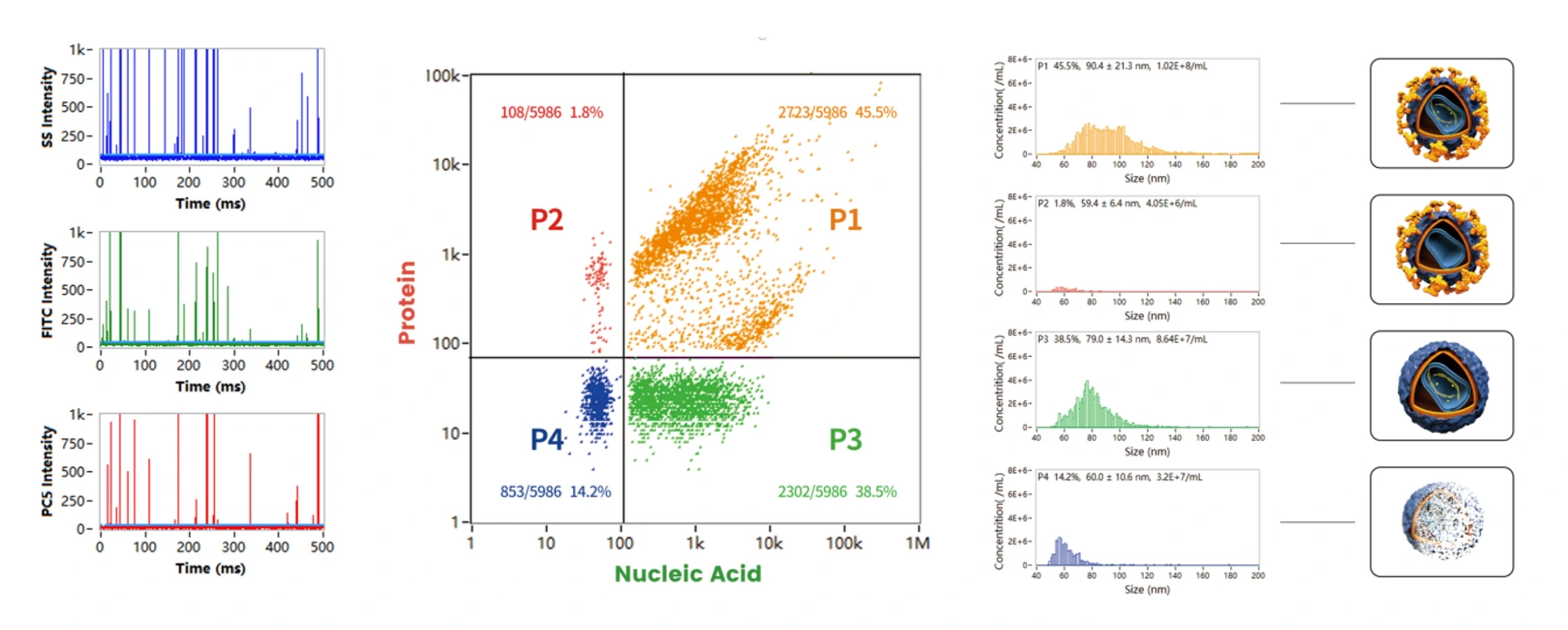
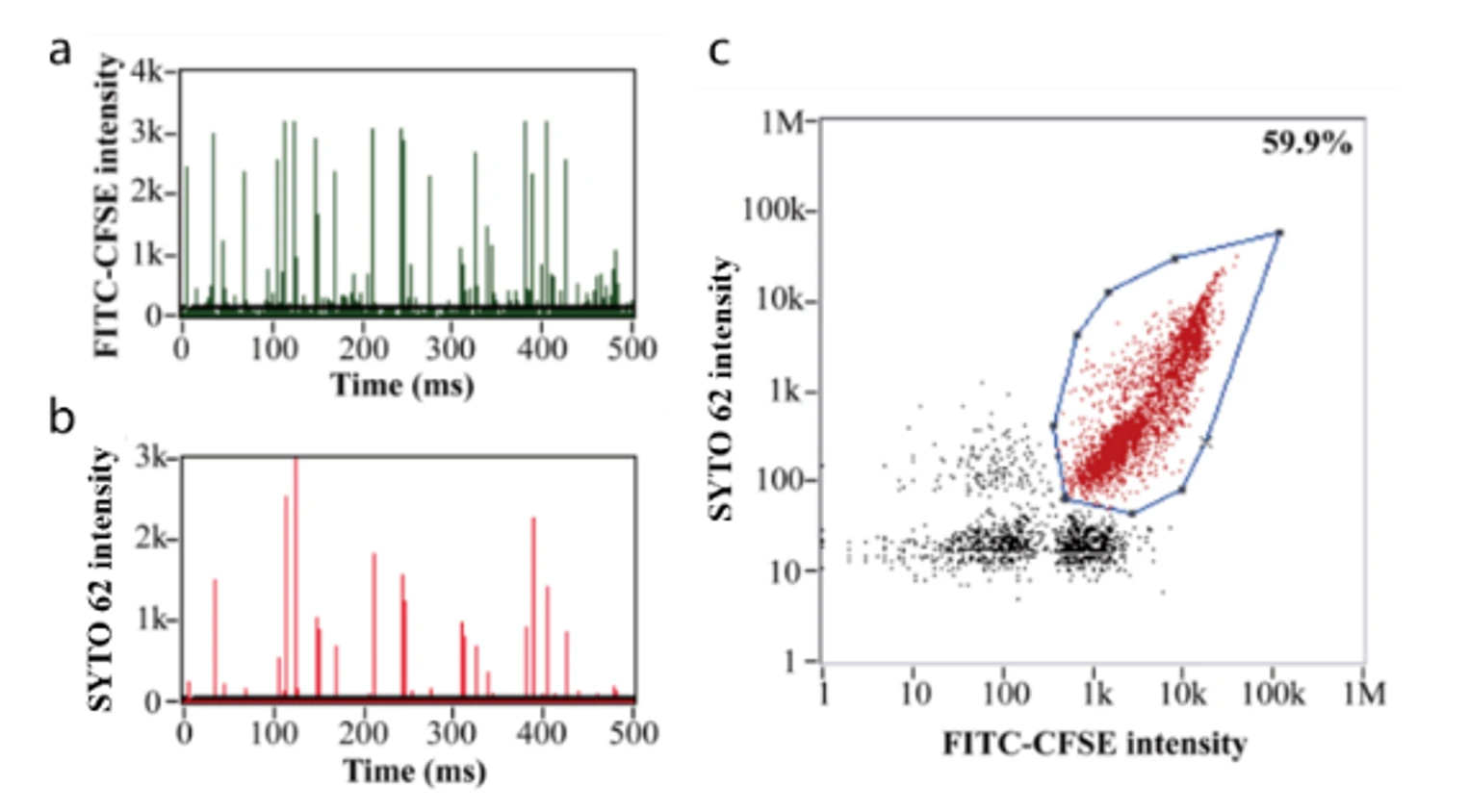
Figure 1. Analyze different compositions of lentivirus particles on the Flow NanoAnalyzer
In Figure 1, the P1 population represents the complete lentivirus, double-positive for labeled nucleic acid and VSV-G proteins, making up ~45% of the particle product, with additional size distribution and concentration data provided in a single 2-3 minute run time. The major particle contaminant is the VSV-G-negative, genome-positive population. Despite the potential for these VSV-G-negative particles to be insufficient for gene integration, they could skew qPCR measurements and particle counting methods.
Copyright © 2022 NanoFCM Inc.