Quantitative Assessment of the Physical Virus Titer and Purity
Rapid quantification of viruses is vital for basic research on viral diseases as well as the clinical application of virus-based products. The plaque assay is the classical method used for virus quantification, yet it requires 1-2 weeks (for mammalian viruses) or 1-2 days (for bacteriophages) to determine the infectious titers. Although the enzyme-linked immunosorbent assay (ELISA) and quantitative polymerase chain reaction (qPCR) can quantitatively measure specific proteins and the nucleic acid sequences of viruses, respectively, the stringency of these "bulk" analytical methods is low. They are incapable of distinguishing proteins originating from complete virions or empty capsids, or soluble viral proteins and viral nucleic acids packed inside the capsid or that are free in solution.
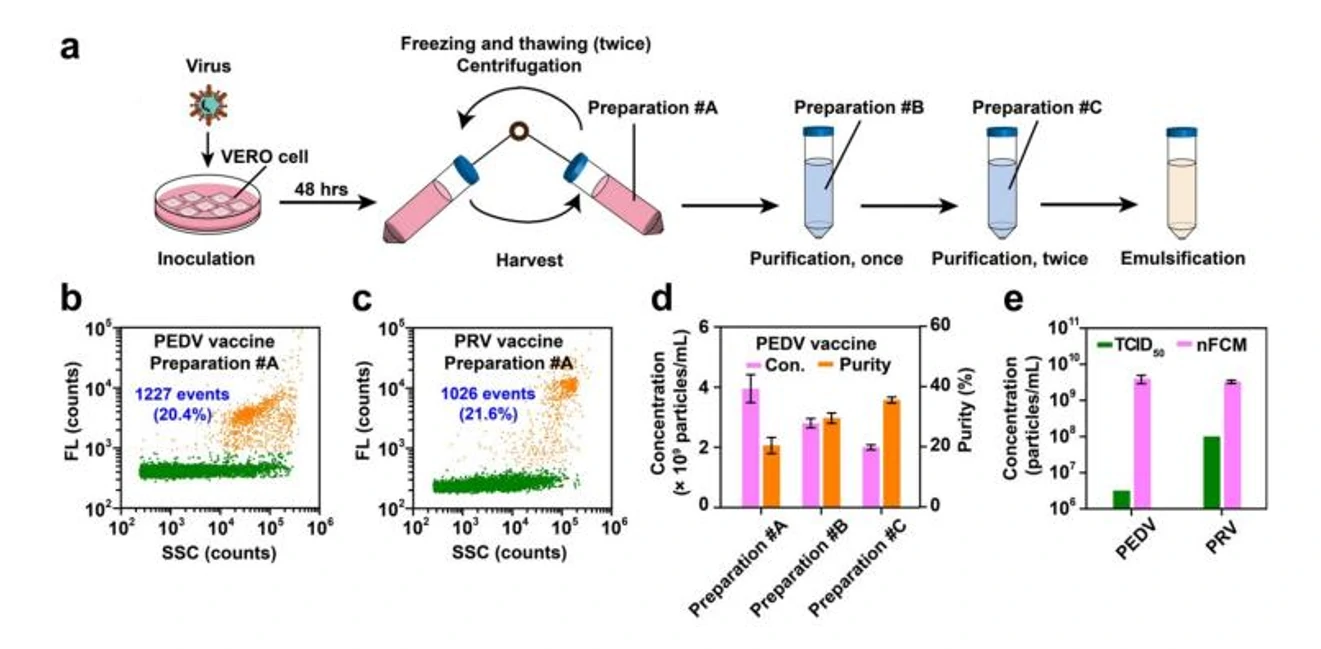
Figure 1. Characterization of virus-based vaccines.
A high-throughput single-particle method to enumerate intact viral particles using the Flow NanoAnalyzer was reported. Using the bacteriophage T7 as a model system, intact virions were completely discriminated from empty capsids and naked viral genomes. Successful measurement of the physical virus titer and purity was demonstrated for recombinant adenoviruses, which could be used for gene delivery, therapeutic products derived from phage cocktails, and infected cell supernatants for veterinary vaccine production.
Angew. Chem. Int. Ed., 2021, 60, 9351-9356.