NanoAnalyzer
- Laser (blue, green, red)
- One SSC & Two FL channels
- Lower detection limit
- High detection limit (1 micron)
- Up to 12,000 particles/min
Single-Molecule NanoAnalyzer
- High-throughput
- Automatic clean-up
- Up to 3 markers
- New Features
Fluorescent quantitation
Nucleic acid copy number per LNP
Copy number of specific proteins
Encapsulation efficiency
Reagents Consumables
- Quality Control
- Size Standard
- Fluorescent quantitation
- Fluorescent Antibody





















EXTRACELLULAR VESICLES

THE ORIGIN AND PHENOTYPING OF EXTRACELLULAR VESICLES

VACCINE

COMPREHENSIVE MEASUREMENT SUITE FOR NANOSIZED DRUG PRODUCTS

NANOMEDICINE

COMPREHENSIVE MEASUREMENT SUITE FOR NANOSIZED DRUG PRODUCTS

BACTERIA DETECTION

TRULY QUANTITATIVE MEASUREMENT OF TOTAL BACTERIAL TITRE
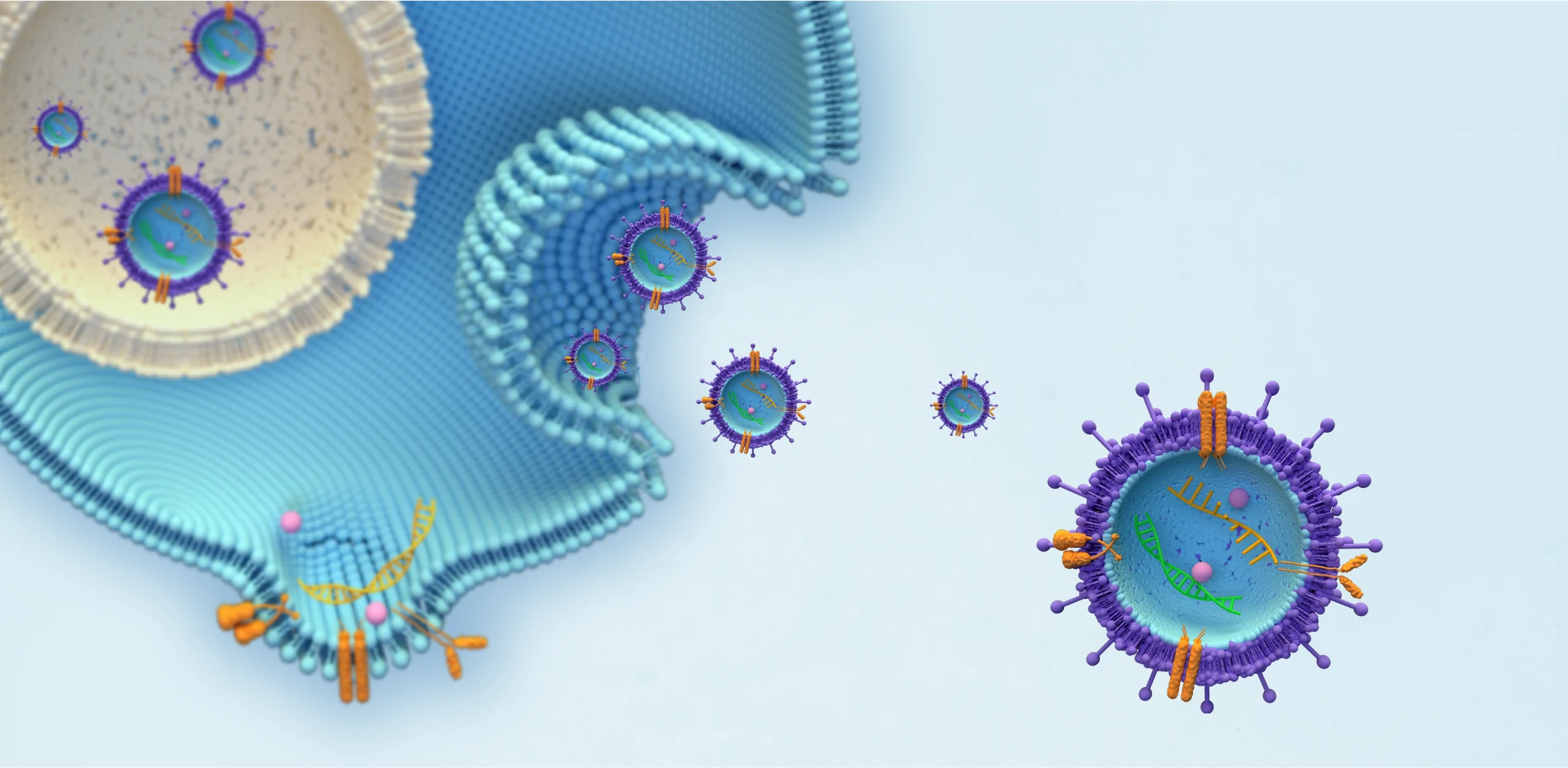
VIRUSES/VIRAL VACCINE

INTEGRATE INTO THE GENOMES OF THE HOST CELLS

OTHERS

COMPREHENSIVE MEASUREMENT SUITE FOR NANOPARTICLES AND INDIVIDUAL MITOCHONDRIA


EXTRACELLULAR VESICLES

THE ORIGIN AND PHENOTYPING OF EXTRACELLULAR VESICLES

VACCINE

COMPREHENSIVE MEASUREMENT SUITE FOR NANOSIZED DRUG PRODUCTS

NANOMEDICINE

COMPREHENSIVE MEASUREMENT SUITE FOR NANOSIZED DRUG PRODUCTS

BACTERIA DETECTION

TRULY QUANTITATIVE MEASUREMENT OF TOTAL BACTERIAL TITRE

VIRUSES/VIRAL VACCINE

INTEGRATE INTO THE GENOMES OF THE HOST CELLS

OTHERS

COMPREHENSIVE MEASUREMENT SUITE FOR NANOPARTICLES AND INDIVIDUAL MITOCHONDRIA

Two Roads to In Vivo CAR-T: The Viral versus Lipid Nanoparticle sprint, Who will win the race? :
CAR-T Therapy continues to evolve rapidly, mRNA encoding CAR constructs directly to T cells in the bloodstream, potentially enabling CAR T cell production in the body without the need to isolate and manipulate T cells outside of the patient. This in vivo CAR-T engineering is a game changer and has significantly reduced the time to patient treatment. Join Joseph Brealey to find out more about in vivo CAR-T reprogramming approaches and how multiple analytics is consolidated into a single workflow, saving time and cost without compromising data quality for your CAR therapy research and development.
Date :
October 2, 2025
Time :
8:00 AM (PT) | 11:00 AM (ET) | 5:00 PM (CET)
30 +
Countries
300 +
Top Users
80 %
TOP 20 Biopharma
1000 +
Publications