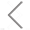EVs isolation from raw milk
Author: admin Date: February 21, 2024
Extracellular vesicles (EVs) have demonstrated unique advantages in serving as nanocarriers for drug delivery, yet the cargo encapsulation efficiency is far from ideal, especially for hydrophilic chemotherapeutic drugs. Besides, the intrinsic heterogeneity of EVs renders it difficult to evaluate drug encapsulation behaviour. Inspired by the active drug loading strategy of liposomal nanomedicines, here we report the development of a method, named “Sonication and Extrusion-assisted Active Loading” (SEAL), for effective and stable drug encapsulation of EVs. Using doxorubicin-loaded milk-derived EVs (Dox-mEVs) as the model system, sonication was applied to temporarily permeabilize the membrane, facilitating the influx of ammonium sulfate solution into the lumen to establish the transmembrane ion gradient essential for active loading. Along with extrusion to downsize large mEVs, homogenize particle size and reshape the nonspherical or multilamellar vesicles, SEAL showed around 10-fold enhancement of drug encapsulation efficiency compared with passive loading. Single-particle analysis by the Flow NanoAnalyzer was further employed to reveal the heterogeneous encapsulation behaviour of Dox-mEVs which would otherwise be overlooked by bulk-based approaches. Correlation analysis between doxorubicin auto-fluorescence and the fluorescence of a lipophilic dye DiD suggested that only the lipid-enclosed particles were actively loadable. Meanwhile, immunofluorescence analysis revealed that more than 85% of the casein positive particles was doxorubicin free. These findings further inspired the development of the lipid-probe- and immuno-mediated magnetic isolation techniques to selectively remove the contaminants of non-lipid enclosed particles and casein assemblies, respectively. Finally, the intracellular assessments confirmed the superior performance of SEAL-prepared mEV formulations, and demonstrated the impact of encapsulation heterogeneity on therapeutic outcome.
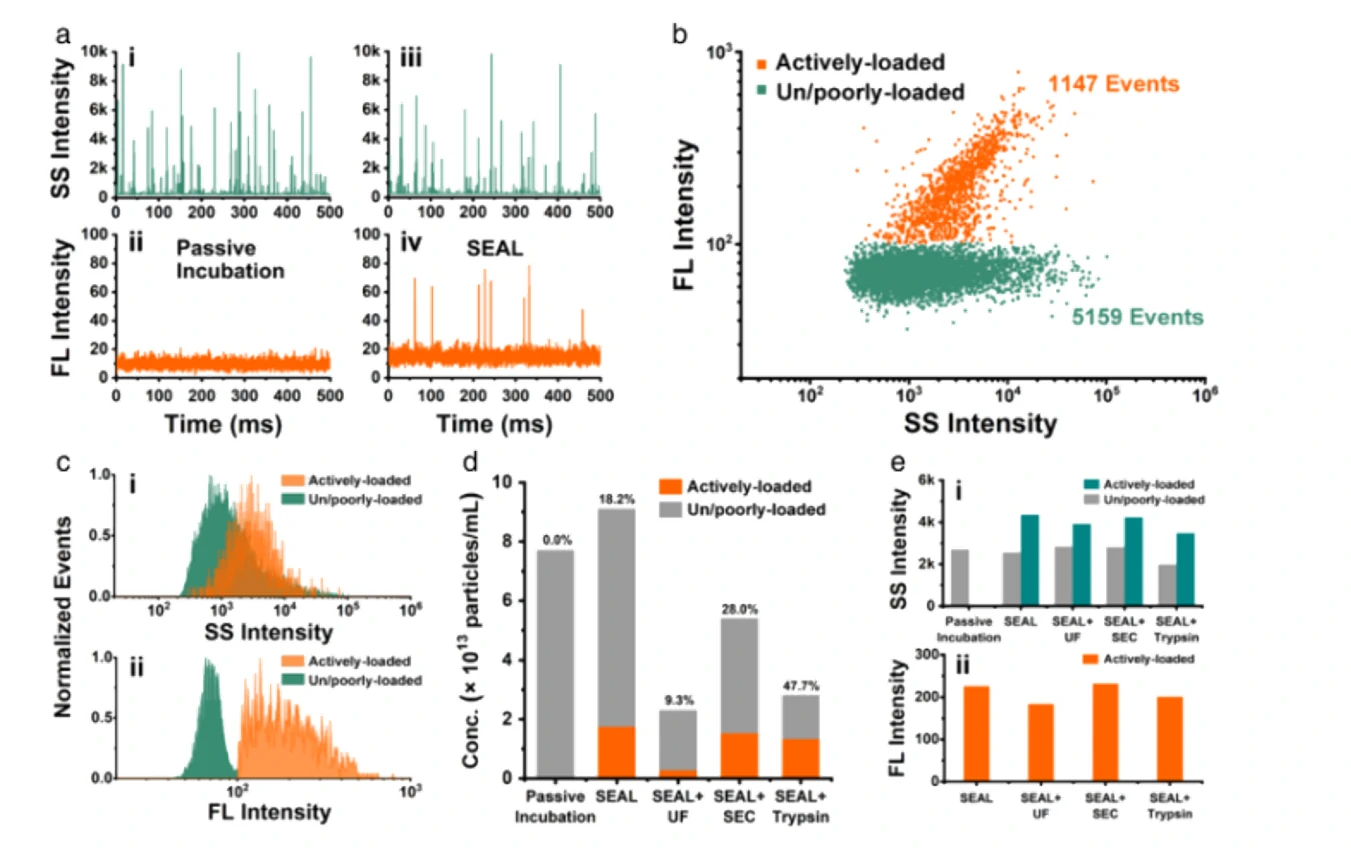
Figure 1.Single particle analysis of the drug loading capacity of SEAL-prepared Dox-mEVs by nano-flow cytometry.
Figure 2. Multi-parameter analysis of the correlation between drug loading capacity and lipid labelling pattern of Dox-mEVs.
The developed cargo-loading approach and the Flow NanoAnalyzer-based characterization method provides instructive insight into the development of EV-based delivery systems.
J Extracell Vesicles, 2021, 10:e12163.