Characterization of Doxorubicin-Carrying Doxoves
Author: admin Date: February 22, 2024
In nanomedicine development, nanoparticles are used as carriers to deliver payloads such as therapeutic agents; thus, the simultaneous detection of both nanoparticles and their cargos is desirable. Doxil (doxorubicin-carrying liposomes) is the first FDA-approved nanomedicine (1995), and DLS and cryo-TEM are the two most commonly used methods for size analysis. While DLS is not suitable for heterogeneous samples, the three-dimensional (3D) reconstruction of cryo-TEM usually takes 2-3 days. Doxoves, a research-grade product of PEGylated liposomal doxorubicin whose physical characteristics and pharmacokinetics are comparable to those of Doxil, were analyzed as a model system. Monodisperse silica nanoparticles are used as the standard to calibrate the size measurement of the Doxoves nanoparticles based on their side-scatter (SS) burst areas. A substantial amount of variation in both size and doxorubicin content can be observed among individual particles.
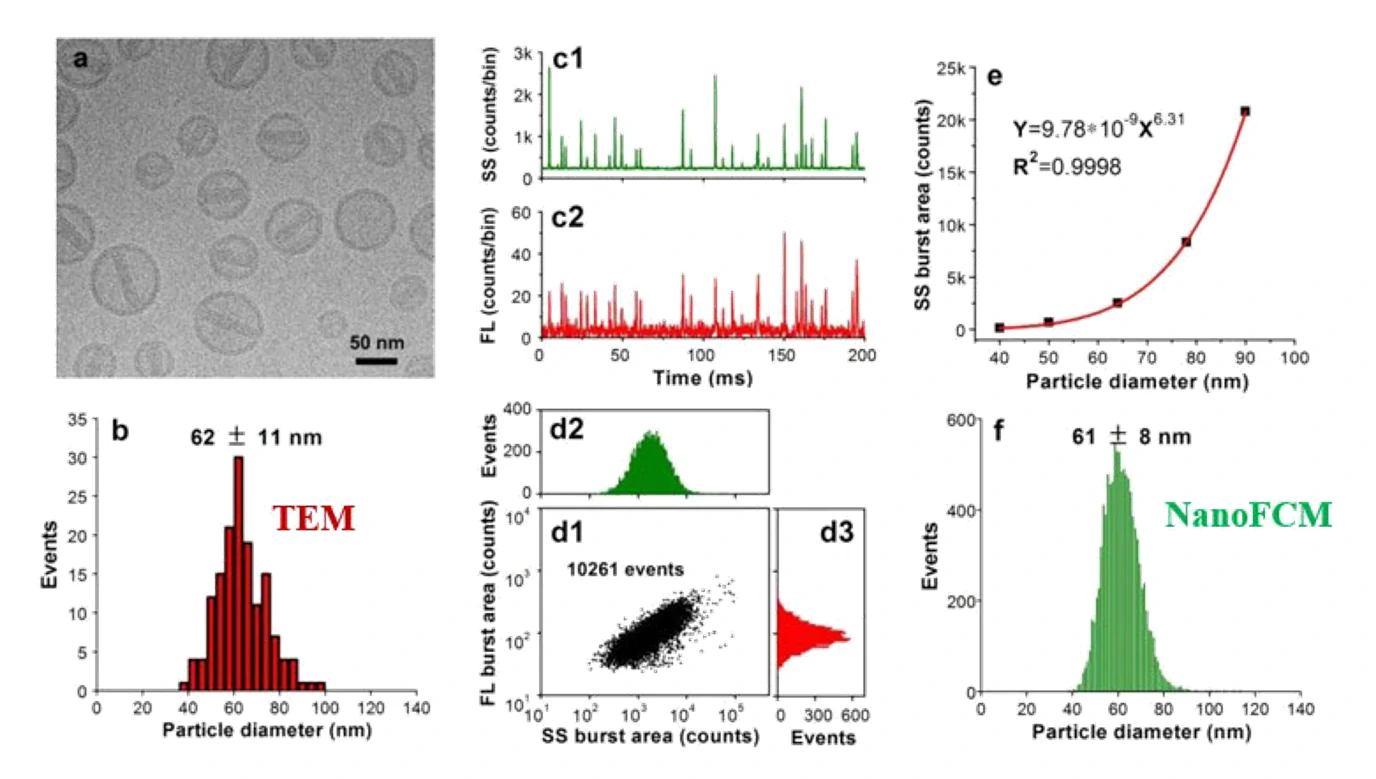
Figure 1. Characterization of doxorubicin-encapsulating Doxoves.
The Flow NanoAnalyzer is sufficiently sensitive to detect both the scattered light and the intrinsic fluorescence of the doxorubicin emitted from individual Doxoves.
ACS Nano, 2014, 8(10),10998-11006.




